In the summer of 2024, Wearables expanded to include patients who receive chemotherapy and/or immunotherapy (chemo/IO)
Goals
Patients who receive chemotherapy and immunotherapy experience a unique set of complications and symptoms associated with their therapy. Our goal of using the wearable device is to obtain heart rate, step count, and sleep data and utilize a machine learning algorithm to help predict complications in chemotherapy and immunotherapy patients. Your participation would be very valuable to help change the course of future patients’ care!
Eligibility Criteria
Patient must be scheduled to undergo IV chemotherapy and/or immunotherapy for a diagnosis of cancer, and cannot be undergoing chemotherapy that is taken by mouth. They must not have started their treatment yet. Patients must be older than 18 years of age.
Virtual Recruitment
We call patients to explain the study background and how to participate. If a patient decides to enroll in the chemo/IO arm, we mail their Fitbit and call them for setup once it’s arrived.
Participation Timeline
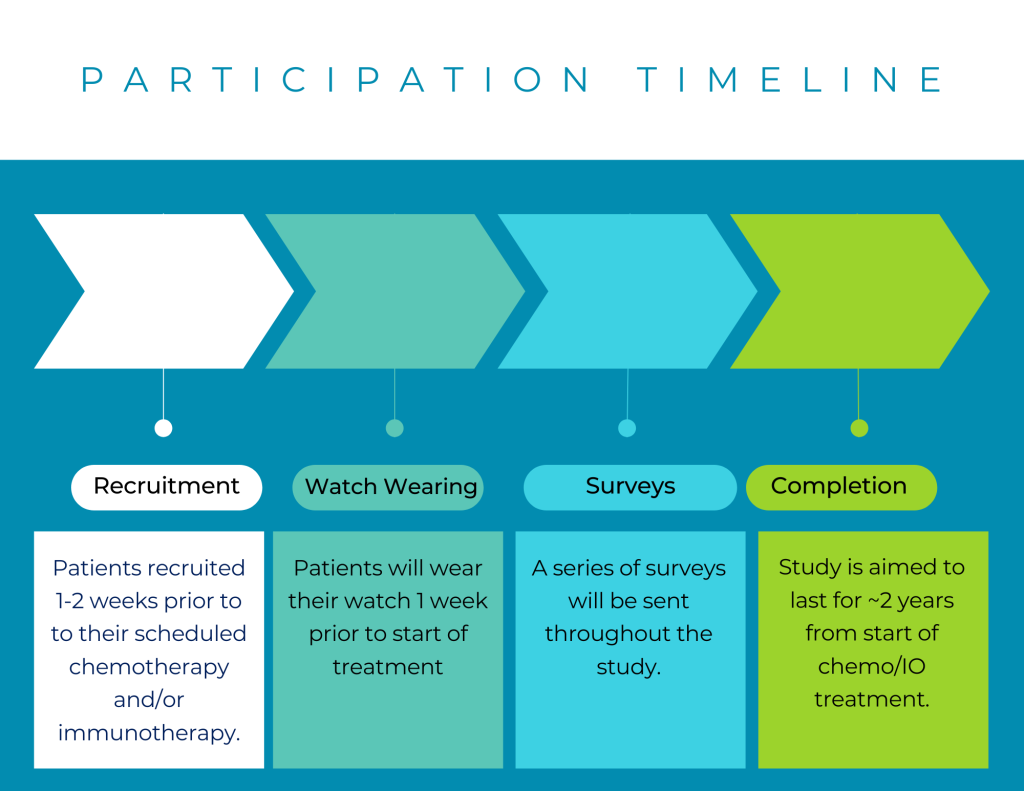
Patients will be recruited 1-2 weeks before their scheduled chemotherapy and/or immunotherapy. They will receive a Fitbit watch and wear the watch 1 week prior to the start of their treatment.
Patients will then have a series of surveys administered throughout the study. The study will be aimed to last about 2 years from the start of their chemotherapy/immunotherapy treatment.